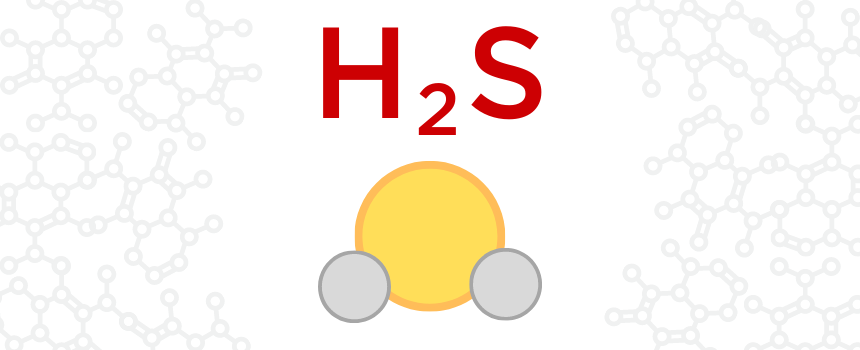
H2S is the chemical formula for the compound, hydrogen sulfide. It is made up of two hydrogen atoms and a single sulfur atom. It is a colorless, flammable, and extremely hazardous gas that has a pungent rotten-egg smell. H2S is produced by the bacterial breakdown of organic materials, as well as animal and human waste. It can also be produced by petroleum/natural gas drilling and refining processes, coke ovens, food processing, kraft paper mills, biofuel generation, and wastewater treatment. In general, H2S can potentially be produced any time elemental sulfur comes into contact with organic material. It can be extremely hazardous, especially in high concentrations. H2S is heavier than air and collects in low-lying and poorly-ventilated areas.
Among the places where H2S creates problems are landfills. The naturally-occurring bacteria that decompose organic waste generate the gas as a byproduct. Such decomposition is an intended feature of landfills, of course, so there’s no way to avoid creating hydrogen sulfide along the way. One thing that differentiates a good, carefully-maintained landfill from a mere trash heap is the way those byproducts are handled. That hydrogen sulfide can pose risks to both the health of people in the vicinity and to the equipment used on site.
Inhalation is the primary vector for human exposure to H2S. Although hydrogen sulfide is known for its awful scent, prolonged exposure will cause a person’s olfactory system to cease registering the smell. This so-called “olfactory fatigue” can even happen with high concentrations of H2S, making it a very insidious substance. Low concentrations can irritate the eyes, nose, or throat, and may cause those with asthma to have difficulty breathing. Long-term effects include headaches, poor memory, reduced attention span, and poor motor function. Inhalation at high concentrations can be even more hazardous and potentially fatal.
It is also important to note that H2S is denser than air, causing it to accumulate at the bottom of confined or poorly-ventilated areas. Special care must be taken when working in areas where the gas might collect. A respirator designed for H2S is usually enough when concentrations are below 100ppm. Above that point, though, more extensive self-contained breathing equipment is necessary.
H2S can also damage equipment – especially gas reservoirs – at landfills and other industrial locations. It acidifies when dissolved in water, and can cause damage throughout the purification process when oxygen and carbon dioxide are also present. Gas reservoirs typically contain CO2 and free water, giving the hydrogen sulfide all the necessary ingredients to become acidic. Corrosive pitting in the machinery can reduce the overall service life of equipment and entire purification systems. In order to prevent damage to equipment, H2S removal medias can be used to draw out the harmful compound before it has the chance to dissolve.
Other equipment is also at risk of corrosion due to this acidification in the presence of oxygen and carbon dioxide. The acidic compound eats away at iron or steel pipes, transforming the surface into iron sulfide scale. Not only does that thin the walls of the piping, but it also introduces flakes of the scaling into the system which may damage delicate equipment downstream.
Additionally, if the H2S is part of a biofuel mixture intended for combustion and power generation, it can be considered a major hazard to the combustion chambers. Electricity generation using biofuels like ethanol and methane biogas is becoming an important part of farmers’ and landfill operators’ business models. H2S is one of the main obstacles they must contend with in order to make their biofuel operations viable. As with pipes, corrosive scaling and pitting of the combustion chamber wall weakens the overall structure of a power generator. The pistons and valves will lose their precise fitting and the generator’s overall efficiency can quickly plummet.
To prevent downstream corrosion, H2S gas can be removed to make the biofuel safe for further usage. This is achieved by passing it through H2S removal media such as FerroSorp. This effectively scrubs the biogas mixture of its hydrogen sulfide content at an early stage, before it comes into contact with more susceptible equipment.
Finally, even if equipment degradation is ignored or no water and carbon dioxide is present in the system, the release or burning of hydrogen sulfide is hazardous on its own. Its chemistry, when combined with atmospheric gasses, ultimately forms sulfuric acid. That, of course, comes back down as acid rain and has disastrous consequences for crops, waterways, and structures alike. Naturally, the precursors of acid rain are carefully regulated.
A proper H2S removal step can bring concentrations down to safe levels, not only protecting equipment, but ensuring the output is well within strict environmental guidelines. The iron hydroxide in the removal media converts H2S into inert elemental sulfur, allowing it to be easily and safely disposed of.
The proper H2S management strategy is critical to any organization that deals with hydrogen sulfide. This hazardous gas has implications for the safety of personnel, the integrity of equipment, and the preservation of the wider environment. Click here for more information about FerroSorp and H2S management strategies using iron-hydroxide-based media.
RESOURCES
CO2 and H2S Corrosion in Oil Pipelines
OSHA FactSheet: Hydrogen Sulfide (H2S)
National Library of Medicine: Hydrogen Sulfide
Health and Social Services Haldimand and Norfolk
The Roles of H2S Gas in Behavior of Carbon Steel Corrosion in Oil and Gas Environment: A Review